Carbonato de sodio United States Pharmacopeia (USP) Reference Standard


Pantoprazole sodium USP Reference Standard Sigma-Aldrich

Cetirizine - USP, PDF, Dangerous Goods

Diclofenac sodium USP Reference Standard CAS 15307-79-6 Sigma-Aldrich

USP Reference Standards

Sodium fluoride USP Reference Standard CAS 7681-49-4 Sigma-Aldrich

PDF) Formulation and in vitro assessment of sustained release matrix tablets of atenolol containing Kollidon SR and carnauba wax
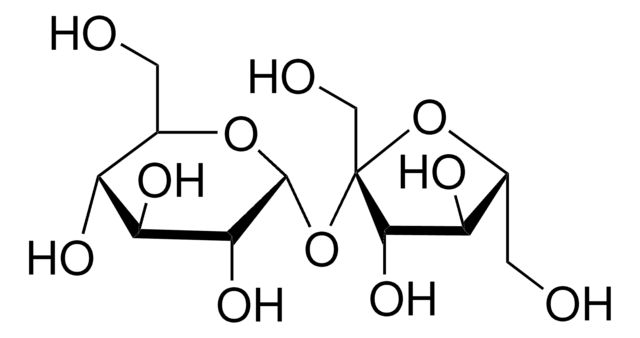
Fructose USP Reference Standard CAS 57-48-7 Sigma-Aldrich

Sodium fluoride USP Reference Standard CAS 7681-49-4 Sigma-Aldrich

Loratadine United States Pharmacopeia (USP) Reference Standard

USP Reference Standards

ES2749800T3 - Naltrexone Sustained Release Formulation - Google Patents

Calcium hydroxide pastes: classification and clinical indications - Fava - 1999 - International Endodontic Journal - Wiley Online Library

PDF) Desenvolvimento e validação de metodologia analítica para quantificação de sódio e cálcio em carbonato de lítio empregando a técnica de ICP OES com amostragem automatizada






